Explain the Difference Between Polar and Nonpolar Bonds
Difference Between Polar and Nonpolar Bonds. To avoid this cancel and sign in to.
Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples
If you are having difficulties explaining something with your own words that is an indication that you dont understand that concept well and you need to go back and study more about itCh.
. Polar molecules occur when there is an electronegativity difference between the bonded atoms. This makes this an angularbent shape of molecule dipole moments wont cancel. Cancellation depends on the shape of the molecule or Stereochemistry and.
Ionic bond full transfer of a valence electron. Which of the following statements is TRUE. Well moreover the polar solvents possess molecules with polar bonds and nonpolar solvents possess molecules with similar electronegativity values.
Difference between polar and non - polar co valent bond. H2O is a polar molecule because of the 2 lone pairs of e- on the central atom O. A polar covalent bond occurs when two atoms share electrons in an unequal manner.
Non-polar colavent bonding electrons are shared equally. Polar covalent Bond formed between the element which have different electronegativity. For instance the bond in chlorine is non.
9 rows Difference between Polar and Nonpolar. Videos you watch may be added to the TVs watch history and influence TV recommendations. Want to see the full answer.
In polar covalent bond the atoms have partial positive and negative charges but in non-polar bonds there are no partial charges for the atoms. This type of bond shares electrons equally unlike polar bonds. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out.
Polar covalent bonds share electrons unequally while nonpolar covalent. Unlike polar bonds non-polar bonds share electrons equally. A covalent bond is formed through the transfer of electrons from one atom to another.
Difference Between Polar and Nonpolar Molecules Net Dipole. A polar molecule always contains polar bonds but some molecules with polar bonds are nonpolar. It is not possible for two atoms to share more than two electrons.
Net dipole is present due to electronegativity differences of participating atoms or. Chlorine contains two chlorine atoms. Explain the difference between a nonpolar covalent bond a polar covalent bond and an ionic bond.
Check out a sample textbook solution. Polar covalent bond. Polar covalent bonds share electrons unequally nonpolar covalent bonds share electrons equally.
Sec 23 25-Explain the difference between polar and non-polar bonds-Distinguish molecular bonds from weak bonds-Explain what makes a molecule either. Single bonds are shorter than double bonds. The rule is that when the electronegativity level is greater than 2 the bond is considered ionic.
Non-polar covalent bonds have no defined axis or axes compared to polar covalent bonds. Polar covalent bonds share electrons equally while nonpolar covalent bonds share electrons unequally. Polar covalent bonds occur when there is a difference in electronegativity or electron affinity between covalently bonded atoms.
Polar colavent bonding electrons shared unequally. Polar covalent bond has a dipole moment whereas a non-polar covalent bond does not. CS2 is a nonpolar molecule because it is linear shaped dipole moments cancel each other out.
A non-polar bond is one in which two or more atoms have the same electronegativity or electronegativities that are less than 04. An example of a non-polar bond is the bond in chlorine. So the electron sharing is not equal.
What is the difference between polar and nonpolar molecules. The molecules in this type of bond also have a defined axis or axes of partial positive and partial negative. 7 rows Within a molecule each polar bond has a bond dipole.
A bond between two atoms or more atoms is non-polar if the atoms have the same electronegativity or a difference in electronegativities that is less than 04. Which statement best explains the difference between polar and nonpolar covalent bonds1 point Polar covalent bonds givetake electrons while nonpolar covalent bonds share electrons. The electron cloud in these molecules are distorted.
Explain the difference between a nonpolar covalent bond a polar covalent bond and an ionic bond. If playback doesnt begin shortly try restarting your device. Electronegativity difference between atoms is.
In nonpolar covalent bonds electrons are shared equally by both members of the bond but they are shared unequally in polar covalent bonds. Learn about chemical bonding how polar covalent bonds form the difference between polar and nonpolar covalent bonds and explore the effects of partial changes. A molecule in which the bond dipoles present do not cancel each other out and thus results in a molecular dipolesee below.
The electronegativity of the atoms involved in a covalent bond determines which will have more pull on the electrons shared between them. When the level is less than 5 it is a non-polar covalent bond. On the other hand non-polar covalent bonds have equal or nearly equal sharing or distribution of electrons between two elements.
The prime difference between polar and nonpolar solvents is the polar solvent gets dissolved in a polar compound whereas the non-polar solvent gets dissolved in non-polar compounds.
Difference Between Polar And Nonpolar Molecules Definition Formation Properties Examples
Difference Between Polar And Nonpolar Bonds
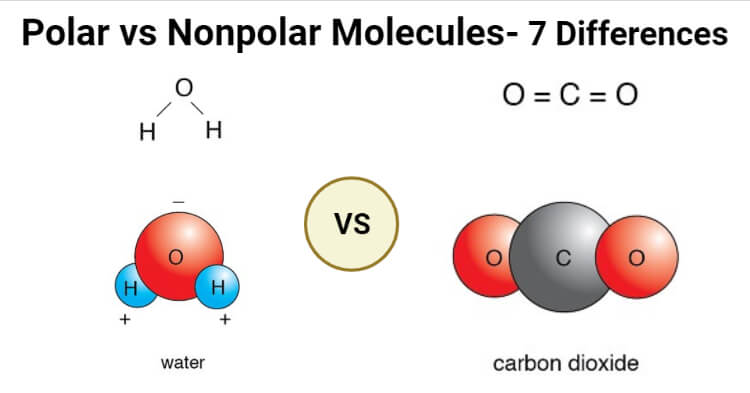
Polar Vs Nonpolar Molecules Definition 7 Key Differences Examples
No comments for "Explain the Difference Between Polar and Nonpolar Bonds"
Post a Comment